Commercial-stage medical device company Avinger Inc. (AVGR:NASDAQ), which designs, manufactures and sells image-guided, catheter-based systems used to treat patients with Peripheral Artery Disease (PAD), today announced the full commercial launch of its Tigereye™ image-guided chronic total occlusion (CTO) crossing system.
Avinger stated that the Tigereye™ system, which it designed and markets, is "the first and only intravascular image-guided, catheter-based system for diagnosis and treatment of PAD." The firm said that as it has now completed its full launch of the Tigereye device it will be readily available for purchase and for use by physicians in surgical procedures in both the U.S. and Germany.
The company advised that it instituted a limited product launch in Q4/20 at a total of 12 clinical centers in Germany and the U.S. The firm reported that around 50 case procedures were then successfully performed in a variety of lesion types and settings, which served to highlight Tigereye's flexibility, reliability and advanced features and capabilities.
Interventional cardiologist and Avinger's Chief Medical Officer Dr. Jaafer Golzar remarked, "The limited launch program demonstrated Tigereye's strong clinical results and efficiency across a wide range of PAD cases...The enhanced imaging, higher rotational speeds and ability to precisely control the device inside the vessel are truly remarkable advancements in Avinger's family of intravascular OCT-guided catheters. Physicians have been able to consistently, safely and effectively cross complex CTOs, including cases that may have required more invasive procedures such as bypass or amputation, fully demonstrating the unique benefits of Avinger's proprietary image-guided technology."
Avinger's President and CEO Jeff Soinski commented, "We are excited to progress to full commercial launch of our next-generation Tigereye device. The limited launch program conducted with 14 physician users affirmed our belief that Tigereye represents an important advance for physicians seeking better solutions on behalf of their CTO patients, who often present with the most challenging and complex PAD cases."
"We believe full commercial availability of the Tigereye device will be an important contributor to expanding our revenue growth opportunities in 2021, both in terms of attracting new Avinger user sites and supporting higher the utilization per site," Soinski added.
The company explained that Tigereye offers "high definition, real-time intravascular imaging and a user-controlled deflectable tip designed for steerability within the lumen." The firm noted that the Tigereye device has a working length of 140 cm and can be used to facilitate treatment in peripheral vessels both above and below the knee and comes with an enhanced distal tip configuration capable of generating rotational speeds up to 1000 RPM allowing it to penetrate extremely challenging lesions.
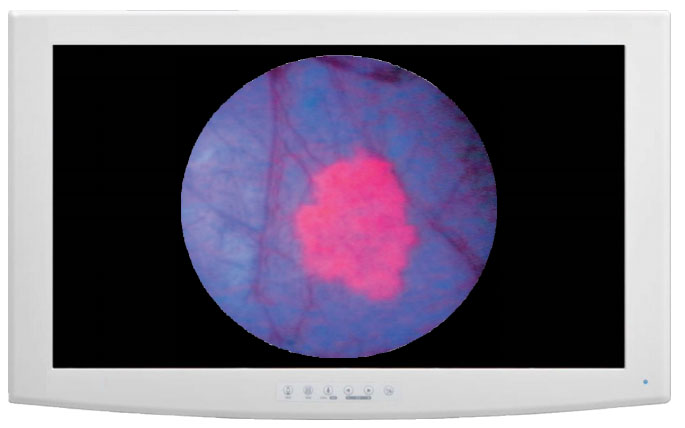
The firm stated that "its proprietary Lumivascular technology allows physicians, for the first time ever, to see from inside the artery during an atherectomy or CTO crossing procedure by using an imaging modality called optical coherence tomography, or OCT, that is displayed on Avinger's Lightbox console." The company added that these advanced features allow surgeons to more safely and accurately navigate their devices and treat PAD lesions from the ability to view real-time OCT images generated from inside the artery.
Avinger is a commercial-stage medical device firm headquartered in Redwood City, Calif. The firm indicated that PAD affects greater than 12 million people in the U.S. and in excess of 200 million globally. The company stated that its Lumivascular platform of product offerings presently includes "the Lightbox imaging console, the Ocelot and Tigereye™ family of CTO catheters, and the Pantheris® family of atherectomy devices."
Avinger began the day with a market capitalization of around $94.3 million with approximately 84.92 million shares outstanding and a short interest of about 1.3%. AVGR shares opened nearly 66% higher today at $1.84 (+$0.73, +65.77%) over yesterday's $1.11 closing price and reached a new 52-week high price this morning of $1.89. The stock has traded today between $1.41 and $1.89 per share and is currently trading at $1.58 (+$0.47, +42.34%).
[NLINSERT]Disclosure:
1) Stephen Hytha compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an independent contractor. He or members of his household own securities of the following companies mentioned in the article: None. He or members of his household are paid by the following companies mentioned in this article: None.
2) The following companies mentioned in this article are billboard sponsors of Streetwise Reports: None. Click here for important disclosures about sponsor fees.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the decision to publish an article until three business days after the publication of the article. The foregoing prohibition does not apply to articles that in substance only restate previously published company releases.
6) This article does not constitute medical advice. Officers, employees and contributors to Streetwise Reports are not licensed medical professionals. Readers should always contact their healthcare professionals for medical advice.