Quite often when I write about companies I'm just about the only one doing so. It's in these situations that Tailwinds can add value by not only highlighting our investment thesis, but in educating investors about a company for which there is little to no publicly available investment opinions.
Provention Bio (PRVB:NASDAQ) is not one of those cases. Instead, this company has a decent market cap of $400 million, has multiple analysts covering the stock and has a marquee investor in Amgen Inc. (AMGN:NASDAQ). At the same time, it has a drug that is going after a very common and well-publicized disease and, with the success shown here, has garnered significant attention from investors and the medical community.
Therefore I will not be writing a lengthy overview on the company nor on their lead drug candidate, teplizumab. Instead, I'm going to write about Provention to explain why I bought the stock recently and why I believe this is one of the most compelling opportunities I've seen of late.
To do so, we'll cover very briefly the market potential of teplizumab, along with the basis of our belief in the drug's success. Then we'll focus on the confluence of events that has created what we believe to be an amazing buying opportunity in the shares.
Delaying, or potentially curing (!) diabetes, is massive
It almost feels Trump-ian to use adjectives like massive when describing investment opportunities. Expansive words get used far too often by investors touting their holdings (I'm certainly guilty). However, if there ever was a disease that could generate massive opportunities for therapies addressing it, diabetes would be that disease. Check out these statistics from the American Diabetes Association:
- Prevalence: In 2015, 30.3 million Americans, or 9.4% of the population, had diabetes; Approximately 1.25 million American children and adults have type 1 diabetes.
- Undiagnosed: Of the 30.3 million adults with diabetes, 23.1 million were diagnosed, and 7.2 million were undiagnosed.
- Prevalence in seniors: The percentage of Americans age 65 and older remains high, at 25.2%, or 12 million seniors (diagnosed and undiagnosed).
- New cases: 1.5 million Americans are diagnosed with diabetes every year.
- Prediabetes: In 2015, 84.1 million Americans age 18 and older had prediabetes.
- Deaths: Diabetes was the seventh leading cause of death in the United States in 2015, with 79,535 death certificates listing it as the underlying cause of death, and a total of 252,806 death certificates listing diabetes as an underlying or contributing cause of death.
Diabetes is one of the most followed and actively researched diseases. It's simple to detect even in prediabetic individuals. A therapy addressing diabetes would have a large market right out of the gate due to its common occurrence and the fact that, to date, no approved drugs have shown any efficacy in preventing, or even delaying, the onset of diabetes.
Teplizumab could be the therapy that wins this market. As Provention said when they released results from their "At Risk" study: "Results from the study showed that a single 14-day course of PRV-031 (teplizumab) significantly delayed the onset and diagnosis of clinical T1D, as compared to placebo, by a median of two years. . .During the trial, 72% in the placebo group developed clinical diabetes compared to only 43% of the PRV-031 (teplizumab) group."
Delaying diabetes by two years is a big deal. "I think everyone would agree that two years without diabetes is a gift. Type 1 diabetes is a disease that's with you literally 24/7. There is not a single activity that someone with type 1 diabetes does that in some way isn't affected by the disease. . .So to not have the disease for two years is actually very important," says Dr. Kevan Herold, professor of immunobiology and internal medicine at Yale University.
With over 1.5 million Americans diagnosed per annum, the market potential for any therapy that can delay the onset of diabetes is in the billions of dollars. Go global and you can double it again. It's tough to put numbers on the drug at this stage, however it's clear that success here could be worth far in excess of $5 billion per annum in sales to Provention. That is truly massive.
Teplizumab is likely to be approved
The data from the recently completed trial was outstanding. Here's what the company had to say about the results:
"This groundbreaking study demonstrates that we can use immunotherapy, specifically PRV-031 (teplizumab), to prevent or significantly delay the onset of clinical type 1 diabetes by at least two years in individuals who will almost certainly progress to clinical disease," said Dr. Eleanor Ramos, Provention's chief medical officer and chief operating officer. "More importantly, approximately 60% of subjects in the study did not develop T1D following only one course of PRV-031 therapy, double the placebo group. Teplizumab is the first immune modulator to show a delay in the clinical onset of type 1 diabetes."
In other words, "It's a very statistically significant finding," said Dr. Herold, quoted in JAMA. Just as importantly, as was noted by the Expert Opinion on Biological Therapy, "Adverse events reported [for teplizumab] so far seem to be mild and of limited duration." The drug not only works, but is safe. Safety frequently drives the speed of an FDA process.
The company already has obtained orphan drug status. Now, on the back of the recent data, the company received breakthrough therapy designation (BTD). This puts them on a much faster track for approval and would seemingly greatly increase the odds of approval for those handicapping the situation.
Here's why BTD is significant. According to Provention, "BTD is an FDA program designed to expedite the development and review of therapeutic candidates intended to treat serious or life-threatening diseases. To qualify for this designation, preliminary clinical evidence has to indicate that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint. The benefits to Provention of this BTD include more intensive and interactive guidance from FDA on an efficient drug development program, access to a scientific liaison to help expedite review time, and eligibility for Priority Review if relevant criteria are met."
In other words, instead of a phase 3 trial, Provention is proceeding, with permission from the FDA, straight into a registration filing. It will take time to file, and for review, but we're now talking months, not years, before teplizumab will possibly be on the market. And, with the outstanding results, robust safety data, a cooperative FDA, and a disease that is high-profile with zero approved therapies, teplizumab would appear to have a high chance of receiving approval on this next go-round with the FDA.
Provention Bio is ripe now
I love when the market misprices securities. In the micro-cap space this happens frequently. News can be misinterpreted or overwhelmed by other factors. This is the case here.
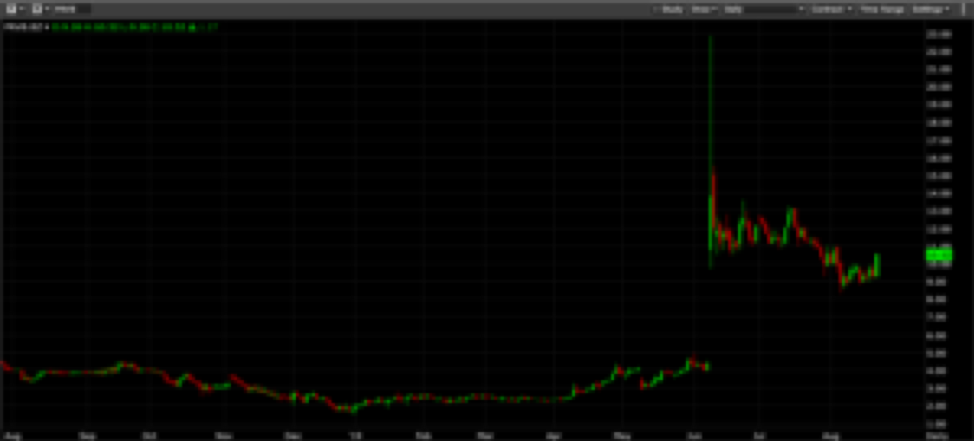
When PRVB released their trial results, the stock ran from the $4 area to over $22 per share. It was off to the races, and those of us not already in the game missed it. . .or so it seemed.
Instead of going straight up to a multibillion market cap, PRVB reversed course. This was initially caused by the company announcing a financing. Happens a lot in stocks that make big moves, right? In this case, it took the wind out of the stock's sails and brought it right back to $12. Still a bargain, but it was going to get cheaper.
The company, under no pressing need for capital, withdrew their offering. They also announced a breakthrough drug therapy designation from the FDA. This puts it on a faster path to approval and should have gotten the stock going again. But it didn’t do so, because, one of the larger holders from years gone by needed cash and chose this time to cashlessly exercise 2.5 million warrants, which they then sold into the market. Despite all the great news, PRVB was back to below $9 per share.
At this time, we have been given a second chance. It's almost as if, on Final Jeopardy, Alex Trebek has asked you, "Are you sure about that answer???" Uh, no Alex, I think I'll erase it and put in the right one now. I was just kidding earlier. . .
It’s a fair counterpoint to say the company might be coming back to the market with a financing. At this valuation, however, I'm not concerned, and any deal would simply bring new investors plus eliminate any overhang.
Instead I'll focus on the fact that PRVB is a stock with a potential blockbuster drug that appears to be on track for approval. The upside is huge. And, due to a confluence of events in the market, it’s at a ridiculous valuation. I'm a buyer.
Daniel Carlson is the founder and managing member of Tailwinds Research Group and its parent company DFC Advisory Services, which is a licensed registered investment advisor (CRD # 297209). Tailwinds is a microcap focused research company that provides research on and consults to over 20 emerging growth companies in the technology and life sciences arenas. DFC Advisory Services is an RIA that manages money dedicated to investing in the companies covered by Tailwinds. For more information on these two companies and their track record, please see www.tailwindsresearch.com. Prior to founding these two entities, Dan spent many years working with small public companies, having been CFO of two public companies and helping finance many others. A 1989 graduate from Tufts University with a degree in Economics, Dan’s formative years in business were spent as an equity trader, first on the Pacific Coast Stock Exchange then on the buyside at several multi-billion dollar firms.
This article was submitted by Tailwinds Research. For more information on Tailwinds Research or on Provention Bio, please visit www.tailwindsresearch.com.
Tailwinds owns stock in Provention Bio. For a complete list of disclosures, please click here.
[NLINSERT]Disclosure:
1) Daniel Carlson: I, or members of my immediate household or family, own shares of the following companies mentioned in this article: Provention Bio. I personally am, or members of my immediate household or family are, paid by the following companies mentioned in this article: None. My company has a financial relationship with the following companies referred to in this article: None. Additional disclosures and disclaimers are above. I determined which companies would be included in this article based on my research and understanding of the sector.
2) The following companies mentioned in this article are billboard sponsors of Streetwise Reports: None. Click here for important disclosures about sponsor fees. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Statements and opinions expressed are the opinions of the author and not of Streetwise Reports or its officers. The author is wholly responsible for the validity of the statements. The author was not paid by Streetwise Reports for this article. Streetwise Reports was not paid by the author to publish or syndicate this article. Streetwise Reports requires contributing authors to disclose any shareholdings in, or economic relationships with, companies that they write about. Streetwise Reports relies upon the authors to accurately provide this information and Streetwise Reports has no means of verifying its accuracy.
4) This article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the interview or the decision to write an article until three business days after the publication of the interview or article. The foregoing prohibition does not apply to articles that in substance only restate previously published company releases.




























































