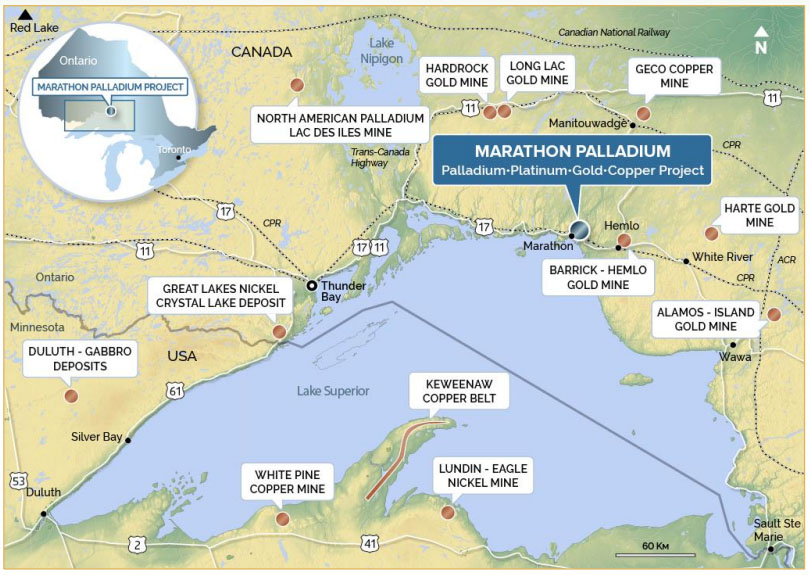
Crucial to electric vehicles (EVs), wind turbines and many other green-tech innovations, these elusive metals aren't as rare as they sound; in fact, you're probably using some right now. Key to a variety of everyday devices, from tablet computers and TVs to hybrid cars, so it may be encouraging to know several kinds are actually common. Cerium, for example, is the 25th most abundant element on earth.
So why are they called "rare" earth elements (REEs)? The name alludes to their elusive nature, as the 17 elements rarely exist in pure form. Instead, they mix diffusely with other minerals underground, making them costly to extract.
A Rare Breed
 Much of the rare earths' appeal lies in their ability to perform obscure, highly specific tasks. Europium provides red phosphor for TVs and computer monitors, for example—and it has no known substitute. Cerium similarly rules the glass-polishing industry, with "virtually all polished glass products" dependent on it, according to the USGS.
Much of the rare earths' appeal lies in their ability to perform obscure, highly specific tasks. Europium provides red phosphor for TVs and computer monitors, for example—and it has no known substitute. Cerium similarly rules the glass-polishing industry, with "virtually all polished glass products" dependent on it, according to the USGS.Permanent magnets are another big role for REEs. Their light weight and high magnetic strength have made it possible to miniaturize a wide range of electronic parts, including many used in appliances, audio/video equipment and military gear. Innovations like small, multigigabyte DVD drives likely wouldn't exist without rare earth magnets, which are often made from a neodymium alloy, but may also contain praseodymium, samarium, gadolinium or dysprosium.
While producing rare earths can cause environmental problems, they have an eco-friendly side, too. They're vital to catalytic converters, EVs and wind turbines, as well as energy-efficient fluorescent lamps and magnetic-refrigeration systems. Their low toxicity is an advantage, too, with lanthanum-nickel-hydride batteries slowly replacing older kinds that use cadmium or lead. Red pigments from lanthanum or cerium are also phasing out dyes that contain various toxins.