Scientists have found and tested an abundant, inexpensive catalyst needed to make hydrogen fuel from sunlight and water. The new catalyst—molybdenum sulfide—is an alternative to platinum, an expensive and rare catalyst used to convert single ions of hydrogen split off from water into hydrogen gas.
"That's the very neat thing here, it is quite inexpensive and abundant," said DOE Chemical Engineer Jens Norskov.
The dream of a hydrogen economy stems from the fact that hydrogen is an energy-dense and clean fuel—upon combustion, it releases water. The problem is that most hydrogen is produced from natural gas in a process that releases carbon dioxide, a greenhouse gas.
Hydrogen-Producing Enzymes
An alternative method is to make hydrogen fuel from sunlight and water. The current process is called photo electrochemical, or PEC, water splitting. When sun hits the PEC cell, the solar energy is absorbed and used for splitting water molecules into hydrogen and oxygen.
Progress, however, in the technology has been limited in part by the lack of cheap catalysts that can speed up the generation of hydrogen and oxygen. Platinum works, it's just expensive and rare.
"We'd like to have something that's cheaper and more abundant," Norskov said.
His team used a theoretical approach to look for hydrogen-producing enzymes—natural catalysts—from certain organisms. This led to molybdenum sulfide.
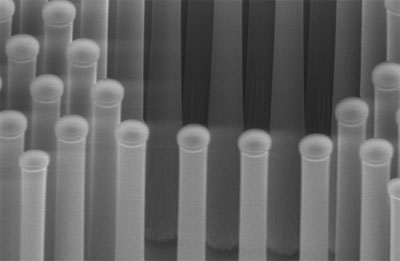
"This actually works quite well," he said. The second part of the research was combining the catalyst with a solar absorber to capture solar energy. It consists of silicon arranged in closely packed pillars, each dotted with tiny clusters of molybdenum sulfide.
When the pillars were exposed to light, hydrogen gas bubbled up as quickly as if the team had used platinum, according to the DOE. A paper describing the research was published last week in Nature Materials.